The pharmaceutical packaging industry is undergoing significant transformation, driven by several key trends that address efficiency, safety, sustainability, and regulatory compliance. As the demand for advanced medicines, such as biologics, vaccines, and personalized treatments, grows, the need for innovative packaging solutions has become more critical. Below are some of the most important trends shaping the future of pharmaceutical packaging.
1. Automation in Pharmaceutical Industry
A major trend in the pharmaceutical packaging sector is the move towards automation. With the increasing demand for faster production speeds, higher operational efficiency, and a reduction in human error, manufacturers are turning to automation to meet these challenges. Equipment featuring cutting-edge technologies, such as machine learning, predictive maintenance, and real-time monitoring, is becoming more common. These automated systems enable pharmaceutical manufacturers to streamline their operations, reduce costs, and optimize production quality.
Machine learning algorithms, for example, can predict potential breakdowns, allowing manufacturers to perform timely maintenance before any critical failures occur. Real-time monitoring systems also provide valuable data that can be used to refine production processes and improve overall efficiency.
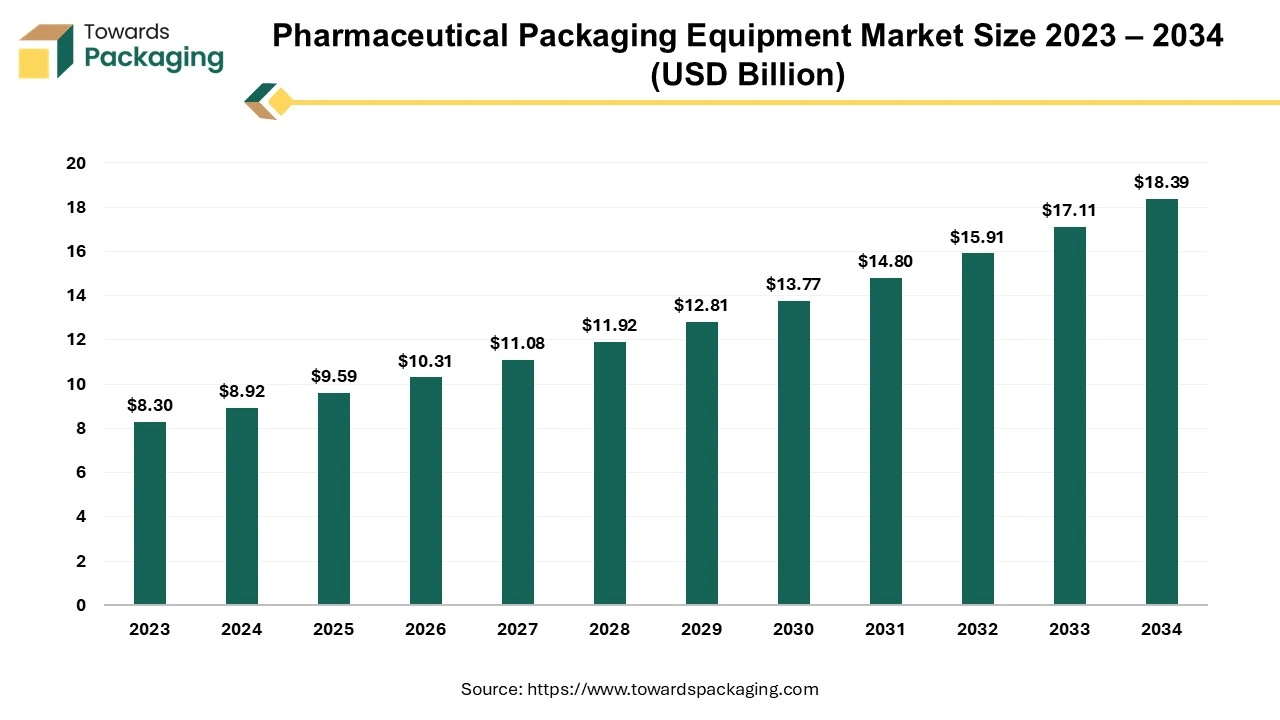
This increasing adoption of automation is reflected in the growth of the pharmaceutical packaging equipment market. Valued at USD 8.92 billion in 2024, the global market is predicted to reach approximately USD 18.39 billion by 2034, expanding at a compound annual growth rate (CAGR) of 7.5%. This growth is driven by the need for more advanced, efficient, and automated packaging solutions to keep up with rising production demands.
Get All the Details in Our Solution – Download Brochure: https://www.towardspackaging.com/download-brochure/5159
2. Growth in Vaccine and Biopharmaceutical Production
The rise of biologics, vaccines, and personalized medicines has fueled a demand for specialized packaging solutions. Unlike traditional drugs, biologics and vaccines are often more sensitive to temperature fluctuations, moisture, and contamination, requiring packaging that ensures the integrity and safety of these products. To accommodate these delicate substances, pharmaceutical packaging equipment is evolving to handle small batch productions, complex formulations, and the increasingly stringent cold chain requirements.
This shift is also driving the need for packaging materials that can maintain the temperature of sensitive products over extended periods. Cold chain packaging solutions, such as insulated containers and temperature-controlled labels, are becoming more essential to protect the efficacy of biologics and vaccines.
3. Rising Shift Towards Eco-friendly and Sustainable Packaging
Environmental sustainability is a growing concern in the pharmaceutical packaging industry. As global awareness of climate change and waste management increases, there is an increasing demand for packaging solutions that minimize environmental impact. Pharmaceutical companies are adopting eco-friendly materials, such as biodegradable, recyclable, or compostable packaging, to reduce waste and lower their carbon footprint.
Additionally, there is a growing interest in reducing the use of plastic and shifting toward more sustainable alternatives like paper-based packaging. Manufacturers are also optimizing their packaging designs to minimize material use, reducing both waste and the environmental impact of production processes. As the industry continues to prioritize sustainability, pharmaceutical companies are focusing on packaging solutions that align with their long-term environmental goals.
4. Serialization and Track & Trace Regulations
The implementation of strict regulatory frameworks is driving the need for enhanced traceability in pharmaceutical packaging. Regulations like the Drug Supply Chain Security Act (DSCSA) in the United States and the Falsified Medicines Directive (FMD) in the European Union require pharmaceutical manufacturers to implement serialization and track-and-trace systems. These measures aim to prevent counterfeit drugs from entering the supply chain and ensure the authenticity and safety of pharmaceutical products.
In response, pharmaceutical packaging equipment is increasingly being integrated with serialization technology. This allows companies to comply with these regulations by incorporating unique identifiers, such as barcodes, RFID tags, or QR codes, on packaging. Serialization systems help track products from manufacturing facilities to the point of sale, offering enhanced security and transparency throughout the supply chain.
5. Smart Packaging
With the growing adoption of digital technologies, smart packaging is becoming an essential tool for pharmaceutical companies. Smart packaging solutions include the integration of RFID tags, QR codes, and temperature sensors, which provide real-time data about the condition and location of products. These solutions help monitor the integrity of sensitive products like biologics and vaccines, ensuring they remain within safe temperature ranges during transit and storage.
Smart packaging also offers consumers and healthcare providers greater visibility into the history of the product, including its origin and handling conditions. This transparency improves patient safety by helping to avoid counterfeit or improperly stored drugs. Furthermore, by leveraging technologies such as IoT (Internet of Things) and blockchain, pharmaceutical companies can achieve higher levels of traceability and ensure compliance with regulatory requirements.
6. Sterile and Single-use Packaging
The demand for sterile and single-use packaging solutions is on the rise, particularly in the context of biologic drugs and parenteral products. Single-use packaging ensures sterility and eliminates the risk of contamination, which is especially important for drugs administered via injection or infusion. This type of packaging also often eliminates the need for additional sterilization processes, reducing both time and cost.
The growing preference for single-use packaging is also being driven by the need to maintain high levels of safety and reduce the risk of cross-contamination in the handling and administration of drugs. With the increasing complexity of biologic drugs, ensuring that each dose is delivered in a sterile and safe manner has become a top priority for pharmaceutical manufacturers.
Invest in Our Premium Strategic Solution: https://www.towardspackaging.com/price/5159
Get the latest insights on packaging industry segmentation with our Annual Membership – https://www.towardspackaging.com/get-an-annual-membership
If you have any questions, please feel free to contact us at sales@towardspackaging.com
Browse our Brand-New Journal:
https://www.towardshealthcare.com
https://www.towardsautomotive.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-packaging/
Get Our Freshly Printed Chronicle: https://www.packagingwebwire.com/


