Table of Contents
ToggleClinical Trial Packaging Market Regional Growth Insights
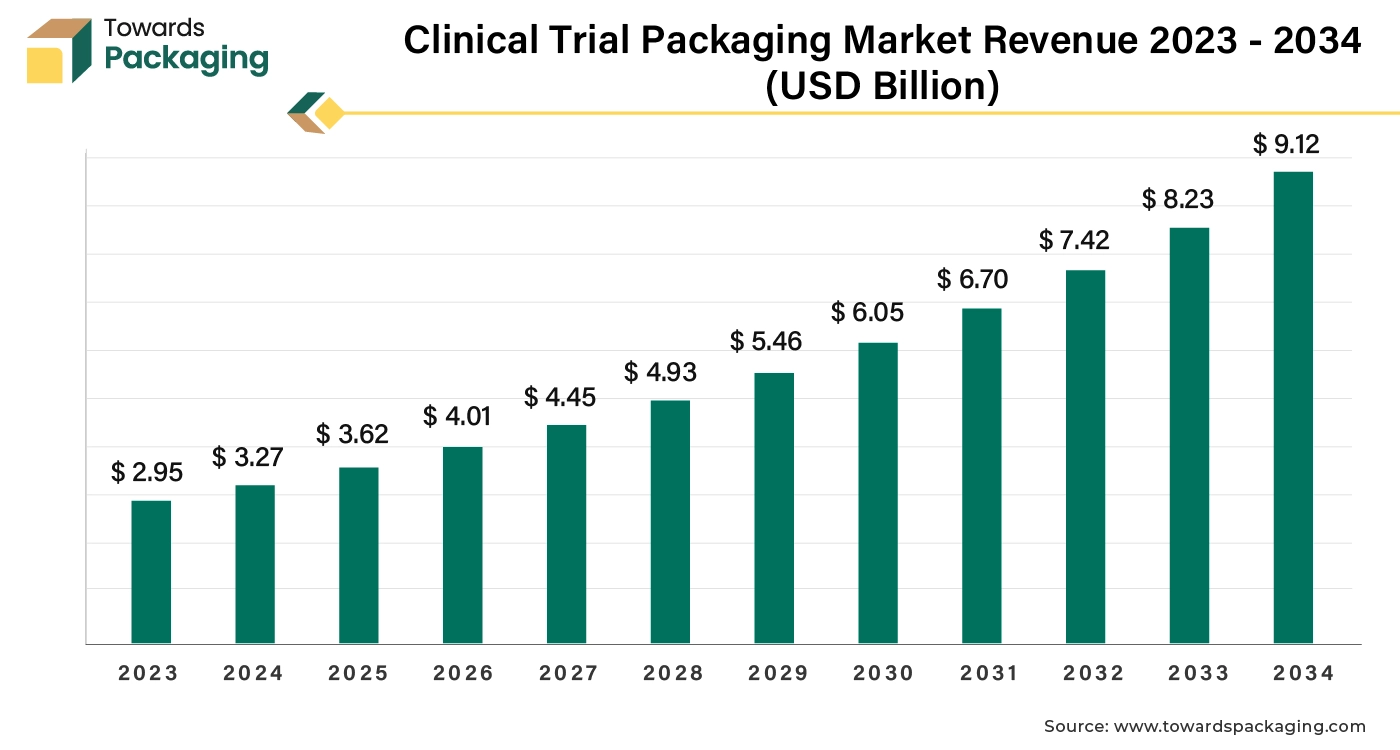
The global clinical trial packaging market was valued at approximately USD 2.95 billion in 2023 and is expected to grow significantly, reaching around USD 9.12 billion by 2034. This growth represents a compound annual growth rate (CAGR) of 10.80% from 2024 to 2034.
Download Statistical Data: https://www.towardspackaging.com/download-statistics/5247
Packaging designed to improve patient adherence, such as user-friendly and informative packaging, is becoming a crucial factor in the clinical trial packaging market. This shift is driving demand for innovative solutions. To stay competitive, key players in the market are increasingly focusing on inorganic growth strategies, such as mergers and acquisitions, to develop advanced clinical trial packaging. This trend is expected to propel the global clinical trial packaging market over the coming years. In the broader packaging industry, the global market size is projected to grow from USD 1.20 trillion in 2022 to USD 1.58 trillion by 2032, with a steady 3.16% CAGR between 2023 and 2032.
Key Insights into the Clinical Trial Packaging Market
- North America led the clinical trial packaging market in 2023.
- Asia Pacific is expected to experience significant growth during the forecast period.
- By clinical trial type, the therapeutic and prevention segment held the largest market share in 2023.
- The bags and pouches segment is expected to grow significantly in the coming years.
- Plastic is projected to dominate material usage in packaging during the forecast period.
- Clinical research organizations were the leading end users in the clinical trial packaging market in 2023.
Clinical Trial Packaging: The Role of Sterile Packaging
Clinical trial packaging plays a vital role in ensuring the success of clinical trials. It involves designing, producing, and labeling the materials that protect investigational products, such as drugs or medical devices, throughout the trial process. Proper packaging ensures that these products are stored, handled, and administered correctly, preserving their integrity and ensuring patient safety.
Compliance with regulatory requirements is a key element of clinical trial packaging, especially guidelines set by agencies like the FDA (U.S. Food and Drug Administration) or EMA (European Medicines Agency). Packaging must meet strict standards regarding labeling, which includes critical information on dosage, administration, storage, and potential side effects. Overall, clinical trial packaging is a detailed and regulated process designed to ensure investigational products are handled safely and effectively throughout the trial.
5 Key Factors Driving Market Growth
- Geographic expansion by key market players is expected to drive growth as companies introduce their brands to new regions.
- A growing focus on cost reduction and production efficiency is further fueling the specialty packaging market.
- The rise of emerging markets and evolving trends in clinical trial packaging is expected to support market growth during the forecast period.
- Increasing regulatory support for clinical trials is anticipated to boost market growth.
- The growing adoption of advanced technologies in clinical trial packaging production will likely accelerate market development in the near future.
- Increased research and development activity, particularly in response to the rise of diseases like monkeypox, is raising demand for clinical trial packaging solutions.
How AI Can Transform the Clinical Trial Packaging Industry
Artificial Intelligence (AI) has the potential to revolutionize clinical trial packaging by automating and streamlining many processes. AI can help reduce time and costs associated with manual tasks like labeling, packaging, and quality control. It can minimize human errors by ensuring consistency and accuracy in labeling and packaging, which is crucial in clinical trials where precision is critical. Additionally, AI can monitor packaging processes in real time, quickly identifying and addressing any issues to maintain high standards of quality and compliance. With AI, packaging processes can be continuously checked for regulatory compliance, flagging potential issues before they become problems. By enhancing efficiency, accuracy, and adherence to regulations, AI is helping make clinical trial packaging more effective and reliable, contributing to smoother and more successful trials.
Buy Premium Global Insight: https://www.towardspackaging.com/price/5247
Get the latest insights on packaging industry segmentation with our Annual Membership – https://www.towardspackaging.com/get-an-annual-membership
If you have any questions, please feel free to contact us at sales@towardspackaging.com
About Us
Towards Packaging is a leading global consulting firm specializing in providing comprehensive and strategic research solutions. With a highly skilled and experienced consultant team, we offer a wide range of services designed to empower businesses with valuable insights and actionable recommendations. We stay abreast of the latest industry trends and emerging markets to provide our clients with an unrivalled understanding of their respective sectors. We adhere to rigorous research methodologies, combining primary and secondary research to ensure accuracy and reliability. Our data-driven approach and advanced analytics enable us to unearth actionable insights and make informed recommendations. We are committed to delivering excellence in all our endeavours. Our dedication to quality and continuous improvement has earned us the trust and loyalty of clients worldwide.
Browse our Brand-New Journal:
https://www.towardshealthcare.com
https://www.towardsautomotive.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-packaging/
Get Our Freshly Printed Chronicle: https://www.packagingwebwire.com/